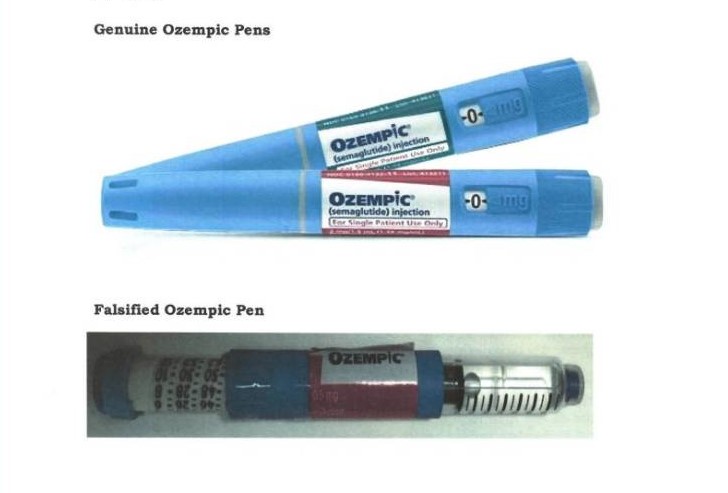
The Pharmacy and Poisons Board (PPB) in Kenya has issued an urgent warning to the public about the presence of counterfeit diabetes medication in the market. The board has specifically raised alarms about falsified Ozempic Pens, which are being misrepresented and sold as genuine diabetes treatment.
Ozempic Pens are used globally to manage type 2 diabetes by helping to control blood sugar levels. However, the counterfeit versions circulating in Kenya have been relabeled from Apidra Solostar Pens, which are intended for treating both type 1 and type 2 diabetes. This mislabeling poses significant health risks to patients who rely on accurate medication for their diabetes management.
The PPB has emphasized that Ozempic Pens are not authorized or registered for use in Kenya. The use of these falsified products could lead to severe health complications, including improper blood sugar management, which can have life-threatening consequences for diabetes patients.
In response to this threat, the PPB has urged the public and healthcare professionals to exercise extreme caution. They have advised against trading, distributing, or administering these counterfeit pens until thorough surveillance and verification processes are completed. The board is working diligently to identify and remove these dangerous products from the market.
The PPB has also called on healthcare providers to be vigilant and report any suspicious diabetes medication to the authorities. Patients are encouraged to verify the authenticity of their medication and consult their healthcare providers if they have any concerns.







Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me. https://accounts.binance.info/vi/register?ref=MFN0EVO1
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Drug information.
where can i buy cheap lisinopril without a prescription
They’re at the forefront of international pharmaceutical innovations.